[最も好ましい] pvt relation for polytropic process 176745-Pvt relation for polytropic process
By Nick Connor A polytropic process is any thermodynamic process that can be expressed by the following equation pVn = constant Definition and characteristics of Polytropic Process Thermal Engineering008 isothermal isentropic_polytropic_process 1 LECTURE UNIT 00 CONSTANT TEMPERATURE or ISOTHERMAL PROCESS (PV = c or T = c) A CONSIDERING A CLOSED OR NONFLOW SYSTEM ILLUSTRATION (Pistoncylinder assembly perfectly cooled) m V Q Pv diagram Ts diagram P T 1 1 2 2 v sEquations 1• the gas undergoes an isentropic process → reversible adiabatic Combining this result with the ideal gas equation of state T 2 T 1 = v 1 v 2 k−1 = P 2 P 1 (k−1)/k The isentropic process is a special case of a more general process known as a polytropic process where → Pvn = constant and n is any number Special Cases n =1 Pv= RT = constant ⇒ isothermal process

Adiabatic Process Relation Between P V And T Testbook
Pvt relation for polytropic process
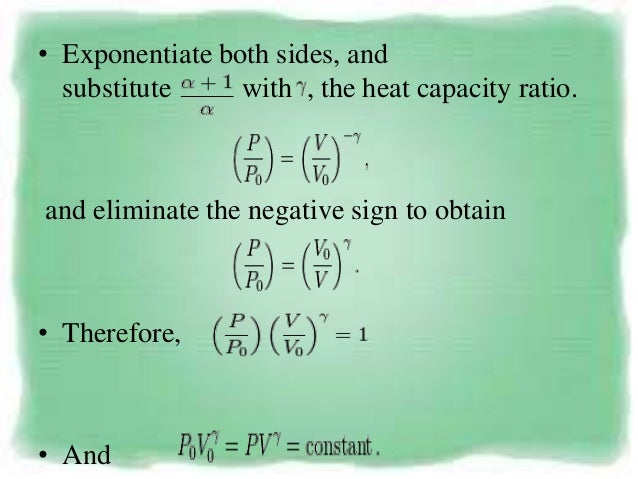
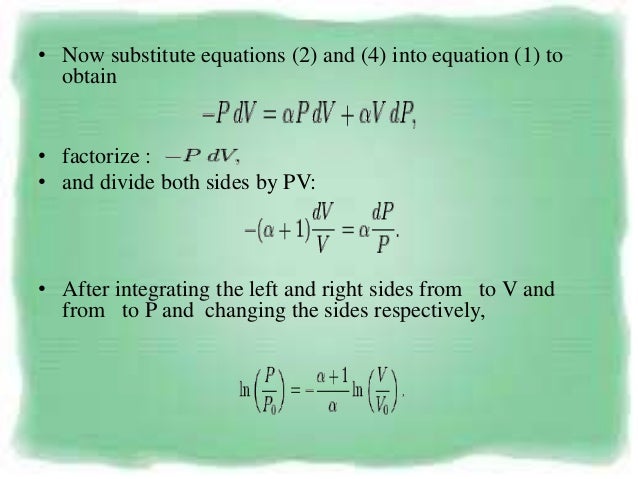
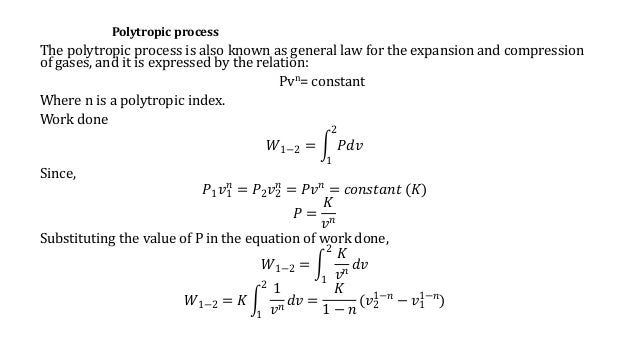
Pvt relation for polytropic process-By comparing this result to the result obtained from the First Law, it is concluded that the polytropic exponent is constant (and therefore the process is polytropic) when the energy transfer ratio is constant for the process In fact the polytropic exponent can be expressed in terms of the energy transfer ratio = where K is negative for an ideal gas This derivation can be expanded toMany processes which occur in practice can be described approximately by an equation of the form PV n = constant, where n is a constant Such a process is called a polytropic process (a) Relation for Polytropic Processes Each of the four processes which have been previously considered is a special case of a polytropic process



Hindi Thermodynamics By Pankaj Kumar Unacademy Plus
I suspect that you mean the specific case of an adiabatic, quasiequilibrium process In that case, set up a thermodynamic cycle composed of an adiabatic expansion followed by an isothermal compression back to the original volume, then followed by an isovolumetric process back to the original stateA polytropic process is a thermodynamic process that obeys the relation PV n = C, where P is pressure, V is volume, n is any real number (the polytropic index), and C is a constant This equation can be used to accurately characterize processes of certain systems, notably the compression or expansion of a gas, but in some cases, possibly liquids and solidsNote that dU = m C V dT, C V = (R/MW)/ ( g 1), PV = nRT and dW = P dV You will arrive at the form (1/T) dT and (1/V) dV on both sides Integrate to obtain the desired result (b) Just use the result from part (c ) along with the ideal gas equation to convert V to P Given in the problem statement
The process z1 →z2 is called irreversible, if the process z2 →z3 = z1 leads to changes in the surroundings, otherwise it is reversible t ,Z t ,Z = Z t ,Z 1 1 2 2 3 3 1 A reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property ofA polytropic process is a thermodynamic process that obeys the relation where p is the pressure, v is specific volume, n is the polytropic index (any real number), and C is a constant All processes that can be expressed as a pressure and volume product are polytropic processes Some of those processes (n=0,1,), are uniqueThis equation can accurately characterize a veryApr 24, 14 · Pressuretime relation It involves polytropic states` knowledge and derivation I am basically an experimentalist I look forward to publish a paper with the help of a physics theoretician I have taken Pt readings, plotted graph on origin software program It clearly shows that with isotropic relations result deviates by about 33% from
The expansion or compression of a gas can be described by the polytropic relation , where p is pressure, v is specific volume, c is a constant and the exponent n depends on the thermodynamic process In our experiment compressed air in a steel pressure vessel is discharged to the atmosphere while the air remaining inside expandsIsobaric and isochoric process, then the corresponding heat capacities are notated by C p and C V respectively These two constants can be related to each other by applying the first law to the following transformation 1 isochoric decrease in temperature and decrease in pressure 2 isobaric increase in volume and increase in temperatureK = CP/CV Here k is the ratio of the specific heat at constant pressure, CP, to specific heat at constant volume, CV The specific heats will be discussed later The boundary work done during the polytropic process is found by substituting the pressure volume relation into the boundary work equation The result is



C P Ds Dt Dv T T Aˆ Az Azz Azœ Azÿ Aˆ Az



Single Stage Air Compressor Basic Theory With Pv Diagram Explanation Marinesite
Calculate Q & W S, in kJ/kg, when ambient air at 104 kPa and 3 K is compressed polytropically to 950 kPa Assume d = 138 for this process path and that air behaves as an ideal gas Read The key to this problem is the fact that the process is polytropic and that the air can be assumed to be an ideal gas Because the process is polytropic, we can determine T 2 and W SThe term "polytropic" was originally coined to describe any reversible process on any open or closed system of gas or vapor which involves both heat and work transfer, such that a specified combination of properties were maintained constant throughout the processPolytropic process is a very general kind of process given by the equation PV^n =constant n can take up any value and depending on that value the process can be classified as a particular process For example, if n=0, the process is isobaric Whereas, if n=gamma which is approximately equal to 14 the process is adiabatic



A Certain Ideal Gas Undergoes A Polytropic Process Pv N Constant Such That The Molar Speci Youtube



Polytropic Processes Definition Examples Diagrams
The polytropic efficiency—also called "smallstage efficiency"—is defined as the isentropic efficiency of an elemental (or differential) stage in the process such that it is constant throughout the whole process The relationship between the isentropic and polytropic compressor efficiency is given by Cohen et al (1999) asPolytropic index A polytropic process is a thermodynamic process that obeys the relation PV^n =C where P is the pressure, V is volume, n is the polytropic index, and C is a constantWhere n is any constant Fig 9 Polytropic Process 1Relation among p V and T from BSBA 5 at Capiz State University Pontevedra Campus



For Polytropic Process Pv N Constant Molar Heat Capacity C M Of An Ideal Gas Is Giv Youtube



Thermodynamic Processes And Equations
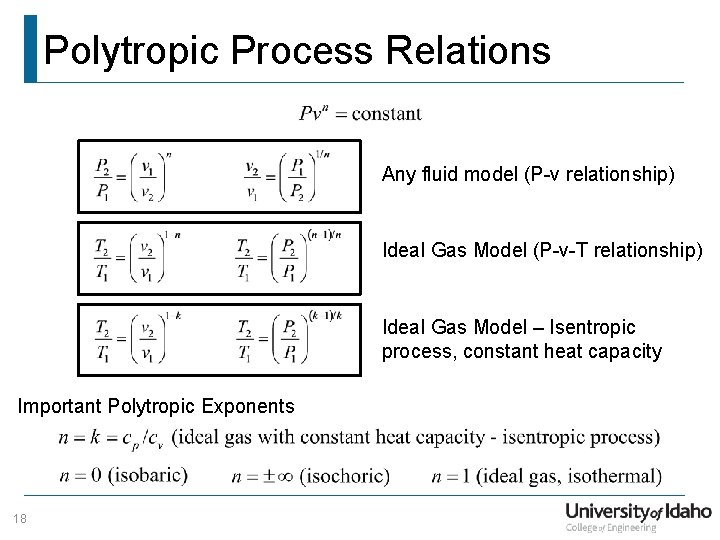
VERY IMPORTANT FOR IES AND GATEFOR MORE INFORMATION PLEASE LOG ON TO wwwgatelecturesmechanicalcomIdeal Bose equation of state The equation of state for an ideal Bose gas is p V m = R T Li α 1 ( z ) ζ ( α ) ( T T c ) α {\displaystyle pV_ {m}=RT~ {\frac { {\text {Li}}_ {\alpha 1} (z)} {\zeta (\alpha )}}\left ( {\frac {T} {T_ {c}}}\right)^ {\alpha }}Analytically One form of this relationship is given by the equation pVn = constant • where n is a constant for the particular process • A thermodynamic process described by the above equation is called a Polytropic process • For a Polytropic process between two states 12 p V p Vn constant 2 2 n 1 1 = =



Isobaric Process Wikipedia



Derivation Of Polytropic Work Formula Youtube
A polytropic process is a thermodynamic process that obeys the relation \( p v^{\,n} = C where p is the pressure, v is specific volume, n is the polytropic index (any real number), and C is a constant All processes that can be expressed as a pressure and volume product are polytropic processes Some of those processes (n=0,1,\gamma,\inftyIn given process dW = 0, dQ < 0 then for a gas 1 Temperature increases 2 Volume decreases 3 Pressure decreases 4 Pressure increasesA polytropic process for an ideal gas is represented by equation = PV^n constant If gamma is ratio of specific heats (Cp/Cv) then value of n for which molar heat capacity of
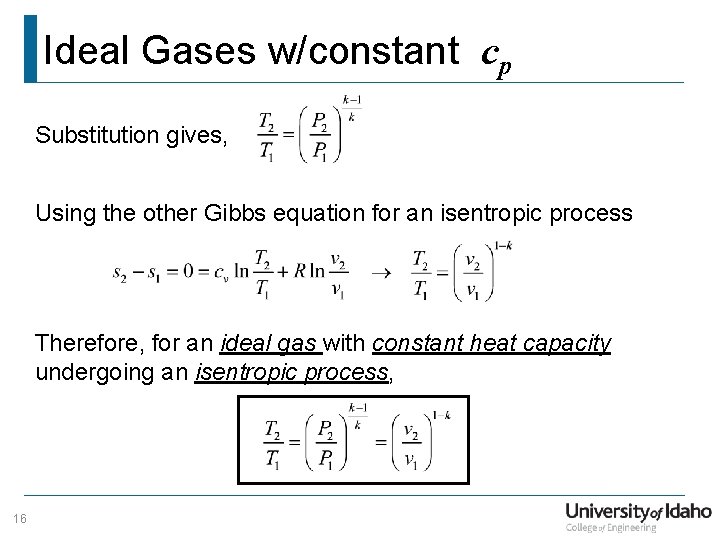


Department Of Mechanical Engineering Me 322 Mechanical Engineering



Single Stage Air Compressor Basic Theory With Pv Diagram Explanation Marinesite
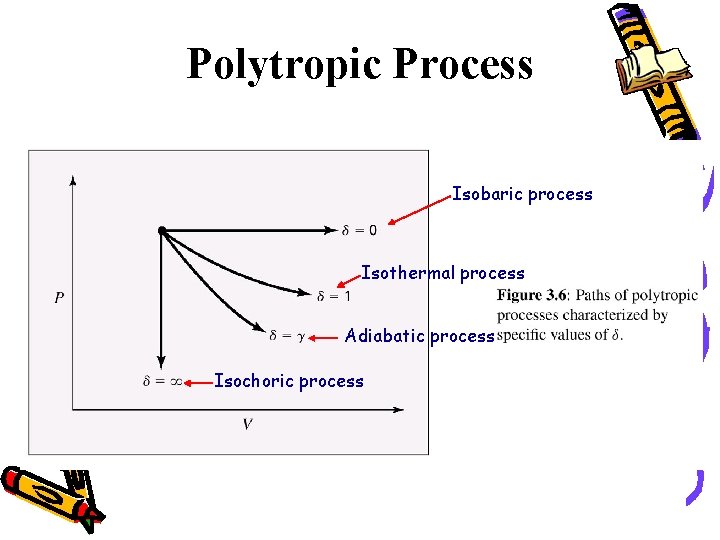
This chapter applies the principles of first law and second law of thermodynamics to compression process The method for the determination of actual work of compressioncon from change in enthalpy is outlined for adiabatic compression case Different approaches for the computation of ideal work reference are then introduced These include isothermal, isentropic and polytropicBecause polytropic processes are general representations of thermodynamic processes, any quantities we calculate for a polytropic process will be a general equation for that quantity in all other thermodynamic processes We proceed to nd the work and heat associated with a polytropic process We start with the de nition of work w= Z V 2 V 1 pdVThe polytropic process equation can describe multiple expansion and compression processes which include heat transfer 1 ISOBARIC PROCESS Pressure is constant PVⁿ = C



Proof Of Pressure Volume And Temperature Ratio Adiabatic Process Youtube



Thermodynamic Properties W Property Table From Direct Measurement
Polytropic process a thermodynamic process that obeys the relation PVⁿ = C where P absolute pressure V – volume n is the polytropic index C constant The polytropic process equation can describe multiple expansion and compression processes which include heat transferWe can determine the shaft work for a polytropic process on an ideal gas using Eqn 1 We can determine T 2 using the folling PVT relationship for polytropic processes Eqn 2 Solve Eqn 2 for T 2 Eqn 3 We can now either evaluate T 2 or use Eqn 3 to eliminate T 2 from Eqn 1 Eqn 4About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators



Adiabatic Process Relation Between P V And T Testbook
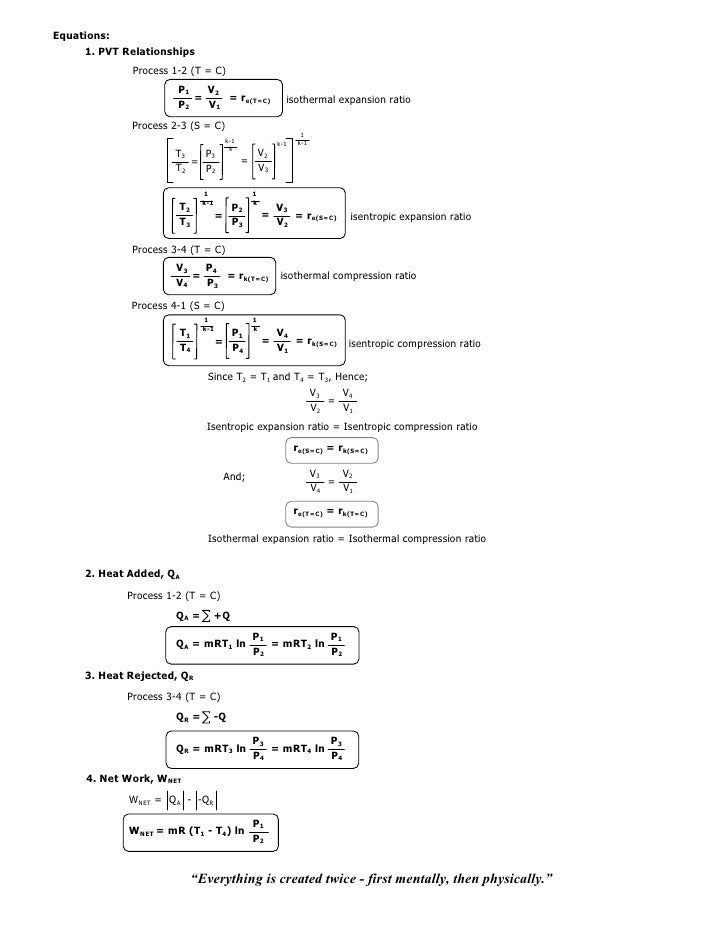


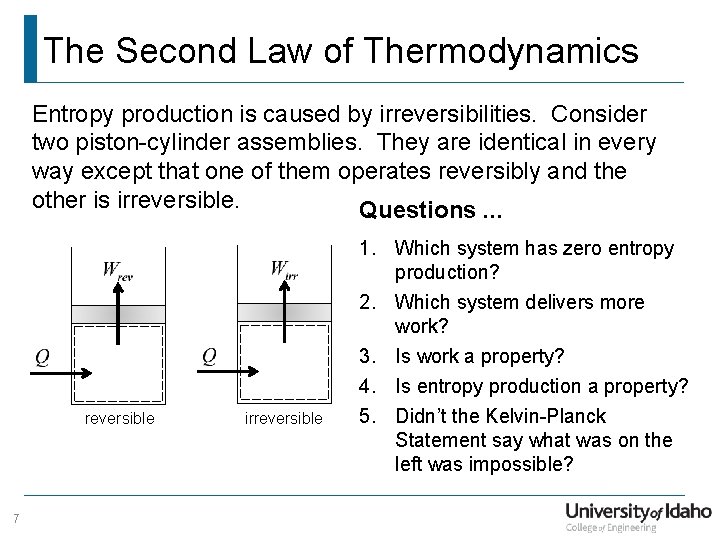
011 Second Law Cycle Analysis
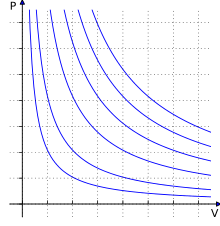
When a gas undergoes a reversible process in which there is heat transfer, the process frequently takes place in such a manner that a plot of the Log P (pressure) vs Log V (volume) is a straight line Or stated in equation form PVn = constant This type of process is called a polytropic processThe basis of this derivation is that the ratio of heat transfer to work (dq/dw the "energy transfer ratio") in a polytropic process is constant Do a Google search on "polytropic energy transfer ratio" and you'll find a link to a useful article in the International Journal of$\begingroup$ Then, do you really mean polytropic process here?



011 Second Law Cycle Analysis
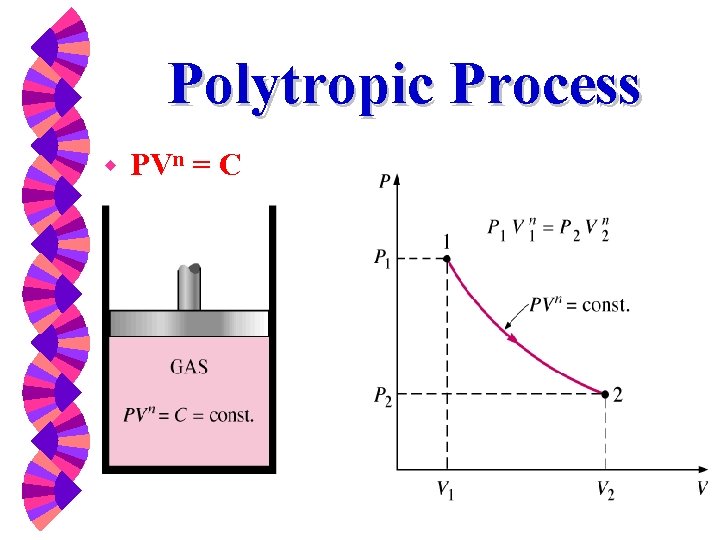


Thermodynamic Properties W Property Table From Direct Measurement
Feb 23, 18 · PVT PVT is abbreviation for Process, Voltage and Temperature In order to make our chip to work in all possible conditions, like it should work in Siachen Glacier at 40°C and also in Sahara Desert at 60°C, we simulate it at different corners of process, voltage and temperature which IC may face after fabricationPV during a polytropic process definition Air (ideal gas with g = 14) at 1 bar and 300 K is compressed till the final volume is onesixteenth of the original volume, following a polytropic process P V 1 25 =const In polytropic process P V η =constantN = polytropic exponent (or index) pVn= constant V2 / V1 = ( p1 / p2 ) (1/n) T2 / T1 = ( p2 / p1 ) (n1)/n (T2 / T1 ) (n/(n1))= P2 / P1 P(1n)* Tn= constant T * V(n1)= constant Cp*dT = Cv*dT R*dT ;



Hw1 1 Pdf Mae 302 002 Thermodynamics Ii Spring Homework Assignment 1 Handed Out Jan 9 Due Problem 1 30 Points Calculate The Missing Course Hero



Thermodynamic Equations Xls
A polytropic process is a thermodynamic process that obeys the relation p v^ {n} = C where p is the pressure, v is specific volume, n is the polytropic index (a real number), and C is a constant The polytropic process equation can describe multiple expansion and compression processes which include heat transferJun 19, 16 · For an isochoric process the work done is always zero A typical example of an isochoric process is addition or removal of heat from a closed system The volume remains constant but temperature and pressure change according to the process thus leading toTo use the relation it is made specific to a path started at known State 1 by i) Selection of a "polytropic coefficient,(n) so that path passes through a specified second state of the gas (Used commonly with industrial experimentation) ii) From the initial state path is selected by specification in accord with constancy of a gas initial state property or interaction condition upon change
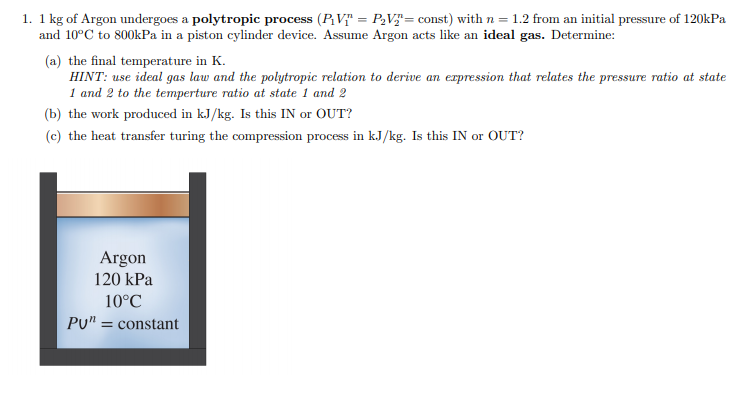


Solved 1 1 Kg Of Argon Undergoes A Polytropic Process P Chegg Com


Ideal Gas Law Wikipedia
Dynamic compression is a polytropic process, meaning that the polytropic index (n) in Eq () is not equal to ratio of specific heats (k) or 10 (n=k would be adiabatic, n=1 would be isothermal) Entropy need not be constant, the process need not be reversible (but it generally is), and heat transfer need not be reasonably close to zeroAdiabatic process Related examples Derivation of the Adiabatic Process formula Work done Adiabatic expansion and compression Adiabatic Reversible and Irreversible Process There are four types of process in a thermodynamic system, which are shown via an image below (image will be uploaded soon)Feb 13, · 3 isentropic process only work transfer no heat transfer but in polytropic heat and work both can be transfer 4politropic index "n" always



Polytropic Process Thermodynamic Derivation Of Polytropic Process Heat Transfer For Polytropic Youtube



271f10l12 Physics Labs
Dec 03, 10 · Relation between PVT Adiabatic process Polytropic process Constant Volume Process Throttling Process Assumptions in Thermodynamic Cycles The analysis of all thermodynamics cycles is based on the following assumptions 1 The gas in



Isentropic Compression Formula Page 1 Line 17qq Com


Pvt Behaviour Of Gases And Relations



Hindi Thermal Physics Iit Jee By Kailash Sharma Unacademy Plus



Isentropic Process Definition Characteristics Nuclear Power Net


The Polytropic Process



Solved Air Is Compressed From An Initial State Of 1 Atm A Chegg Com



Hindi Thermodynamics By Pankaj Kumar Unacademy Plus



Thermodynamic Relationship An Overview Sciencedirect Topics



Adiabatic Process P V T Relation Youtube



Chapter8 Lesson B Polytropic Compression Of Air


Pvt Behaviour Of Gases And Relations


Department Of Mechanical Engineering Me 322 Mechanical Engineering


Ert 64 Thermodynamics Chapter 2 The First Law



Polytropic Process Solved Examples What Is Polytropic Process Definition



What Is Polytropic Process Definition



Exp Polytropic Process For Ideal Gas In Isothermal Condition Gases Pressure
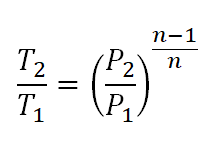


Overview On Thermodynamic Processes With Review Problem English Units Polytropic Process Steemit



A Sketch Of Set Of Four Processes Involved In A Closed Thermodynamic System Is Shown In Homeworklib


What Is Polytropic Process Quora



Derivation Of The Equation Of The Work Done In A Polytropic Process Engineering Stack Exchange



Thermodynamics For Gate By Anuj Kapoor Unacademy Plus



Ch Thermodynamics By Career Avenues Issuu



Adiabatic Process Wikipedia



Change Of Entropy During Polytropic Process Youtube



Adiabatic Process Relation Between P V And T Testbook



Chapter 7 Entropy A Measure Of Disorder Study Guide In Powerpoint To Accompany Thermodynamics An Engineering Approach 6th Edition By Yunus A Cengel Ppt Download



Thermal Engineering Thermodynamics Process Of A Perfect Gas


Ert 64 Thermodynamics Chapter 2 The First Law


What Is Polytropic Process Quora


Thermodynamics



Thermofluids March 19


Adiabatic Process Wikipedia
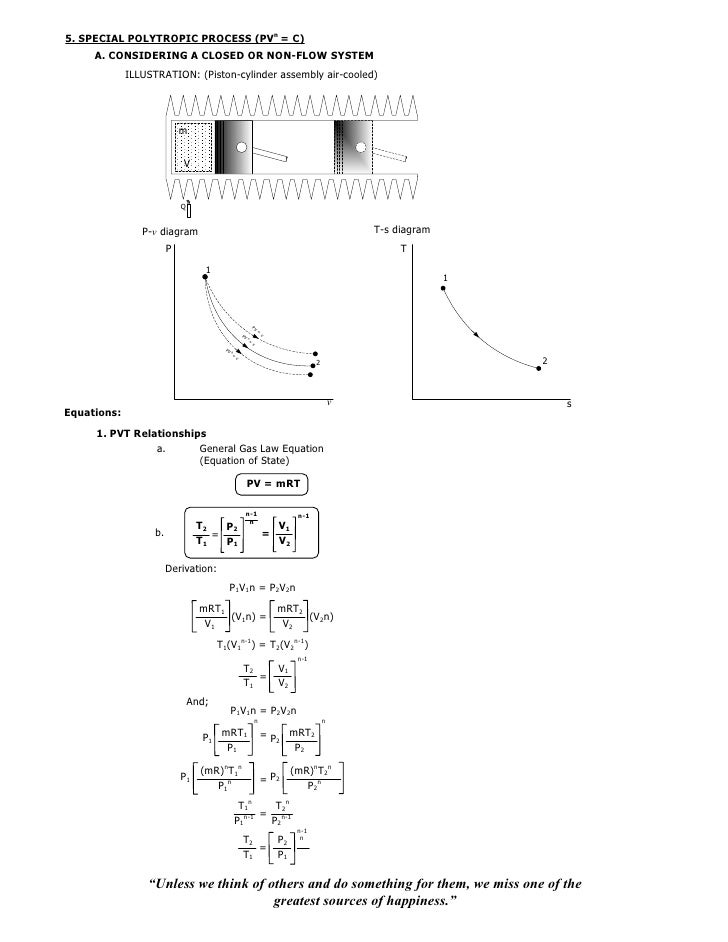


008 Isothermal Isentropic Polytropic Process



Polytropic Process Of An Ideal Gas
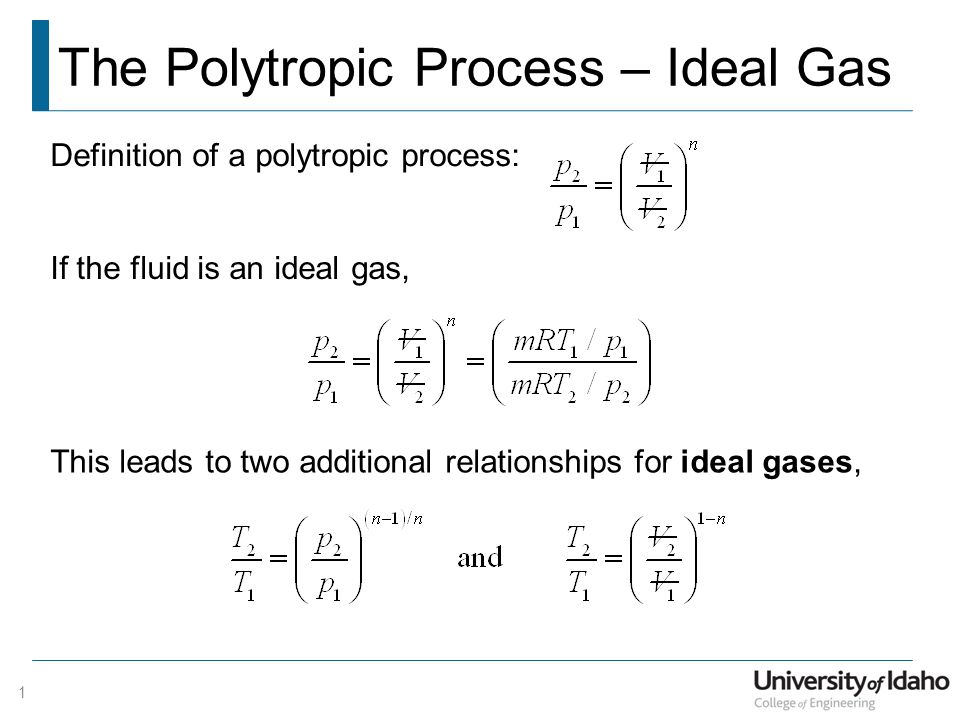


The Polytropic Process Ideal Gas Ppt Video Online Download



3 Ideal Gas Polytropic Processes Thermo Spoken Here
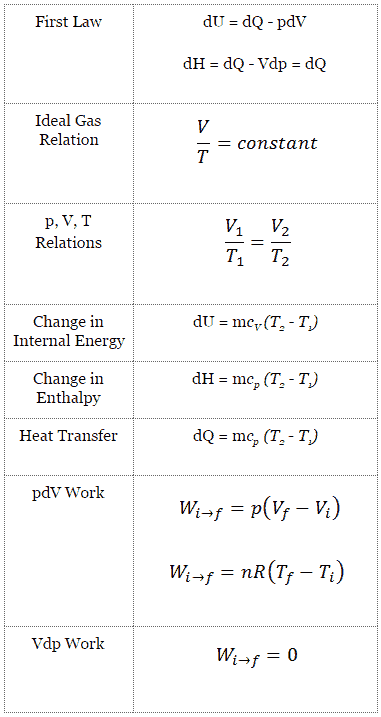


What Is Isobaric Process Definition
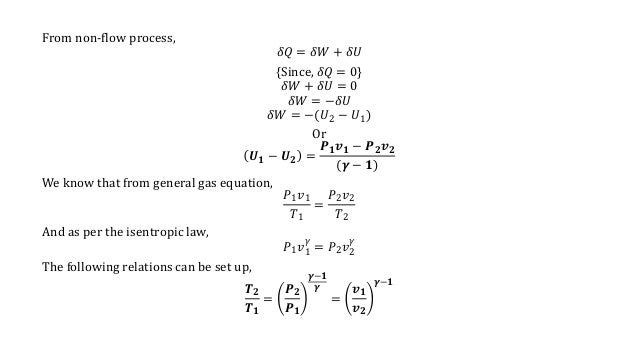


Thermodynamics
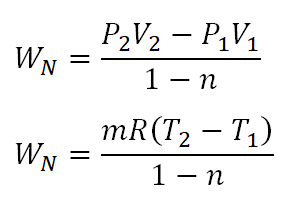


Overview On Thermodynamic Processes With Review Problem English Units Polytropic Process Steemit



Polytropic Processes Definition Examples Diagrams



For Polytropic Process Pv N Constant Cm Molar Heat Capacity Of An Ideal Gas Is Given By



Shortcuts To Convert P V Diagram Into T S Diagram Exergic



Thermodynamics Boundary Work Polytropic Process Youtube



Isochoric Process Ideal Gas Equation Pv Diagram Nuclear Power Net



The Polytropic Process Ideal Gas Ppt Video Online Download



P V Graph For An Ideal Gas Undergoing Polytropic Process Pv M Constant Is Shown Here Find The Value Of M



Why Is Pv Gamma Constant In An Adiabatic Process Physics Stack Exchange



Polytropic Process Definition Characteristics Nuclear Power Net



Polytropic Processes Definition Examples Diagrams


What Is Polytropic Process Quora
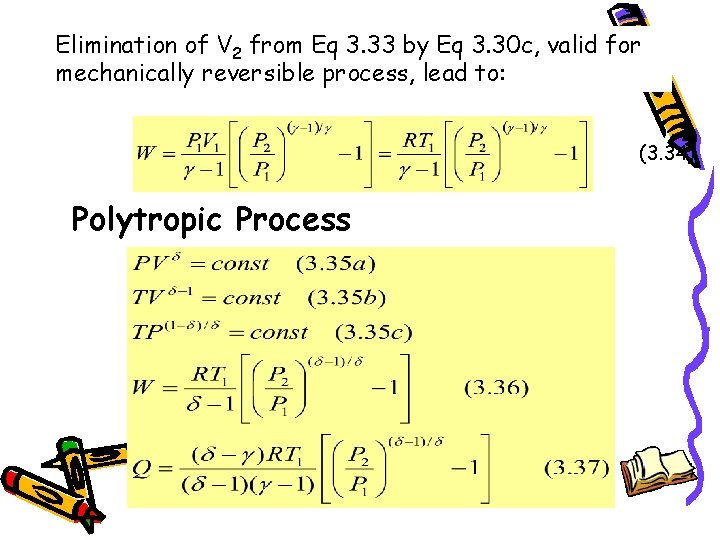


Ert 64 Thermodynamics Chapter 2 The First Law


What Is Polytropic Process Quora
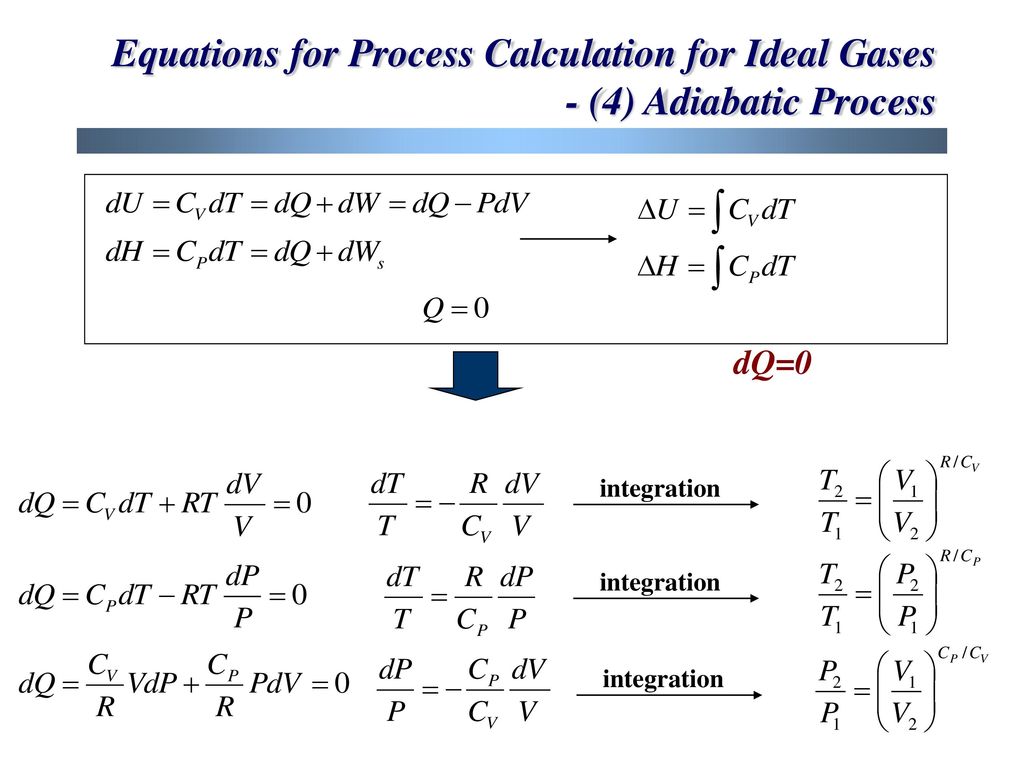


공정 열역학 Chapter 3 Volumetric Properties Of Pure Fluids Part 2 Ppt Download



Isobaric Process Wikipedia



A Sketch Of Set Of Four Processes Involved In A Closed Thermodynamic System Is Shown In Homeworklib



Polytropic Process



Pv Diagrams For Thermodynamic Cycles And Polytropic Processes Youtube



008 Isothermal Isentropic Polytropic Process



Thermodynamics Lecture 10 Polytropic Processes Youtube
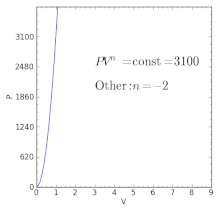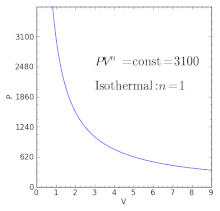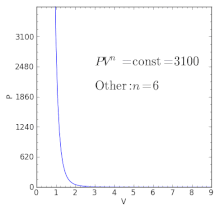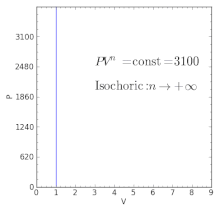


Polytropic Process Wikipedia



Hw1 1 Pdf Mae 302 002 Thermodynamics Ii Spring Homework Assignment 1 Handed Out Jan 9 Due Problem 1 30 Points Calculate The Missing Course Hero
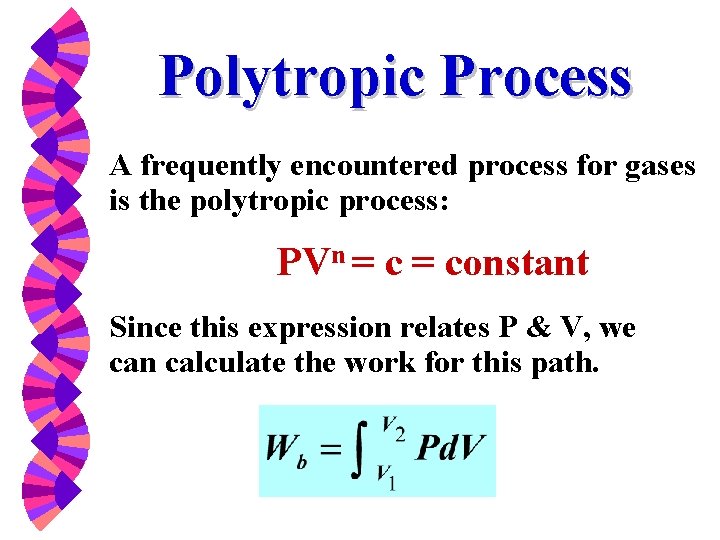


Thermodynamic Properties W Property Table From Direct Measurement



Engineering Thermodynamics By Chetankumar Mohane Unacademy Plus



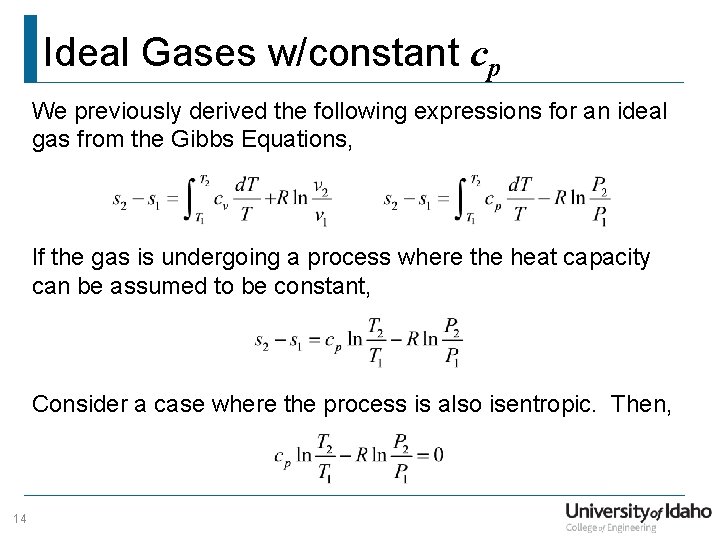
Chapter7 Lesson E Pvt Relationships For Isentropic Ig Processes



Shortcuts To Convert P V Diagram Into T S Diagram Exergic
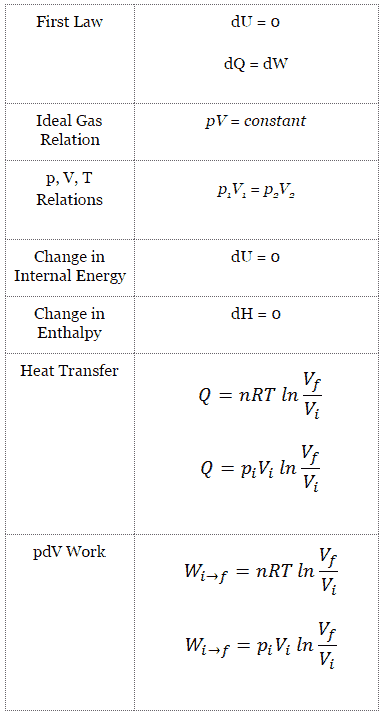


What Is Isothermal Process Definition



Polytropic Processes Definition Examples Diagrams



Polytropic Processes For An Ideal Gas Youtube



Shortcuts To Convert P V Diagram Into T S Diagram Exergic


Department Of Mechanical Engineering Me 322 Mechanical Engineering



Adiabatic Process Relation Between P V And T Testbook


Department Of Mechanical Engineering Me 322 Mechanical Engineering



Adiabatic Process P V T Relation Youtube


コメント
コメントを投稿